Import Medical equipment to Vietnam
Do you want to export Medical equipment to Vietnam? Do you need to calculate freight from the export warehouse to Vietnam? You want to know about Vietnam’s procedure and import duties for importing Medical equipment to Vietnam? Import procedures for Medical equipment?
In this article, Logistics HP Global Vietnam, whose many years in providing freight and logistics services import Medical equipment from many countries to Vietnam, will share knowledge and advice on the above issues.
Import duty of Medical equipment in Vietnam
When importing Medical equipment to Vietnam, the importer needs to pay
- Import value-added tax (VAT)
- Import duty
VAT on medical equipment is 0 – 10%. For detailed conditions for enjoying the 0% and 5% VAT on imported medical equipment, see the article: Duty on importing medical equipment into Vietnam
Current preferential import tax rates for medical equipment range from 0-25%.
In the case of medical equipment imported from countries that have FTAs with Vietnam, they may enjoy Special preferential import taxes if they meet all the conditions set out in the agreement. You should pay attention to this content to enjoy the legal benefits of tax incentives. Currently, Vietnam has signed FTAs with over 50 countries, so it is likely that the goods you import will enjoy Special preferential import tax.
Governmental management on importing Medical equipment to Vietnam
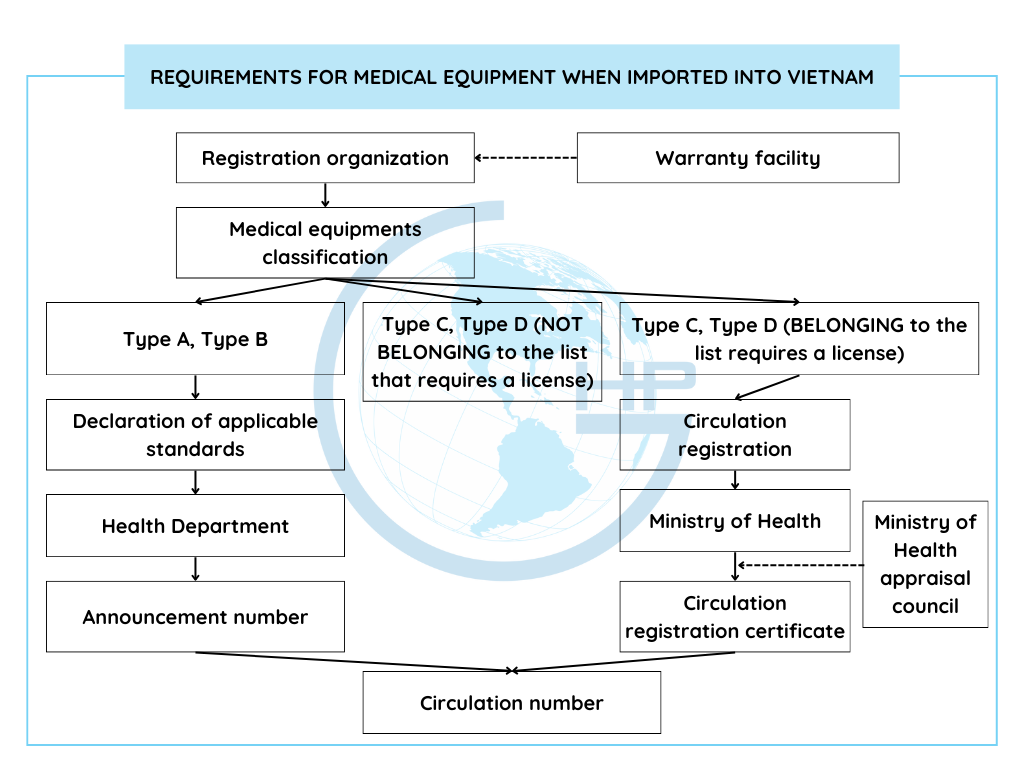
According to Decree No. 98/2021/ND-CP of the government on Medical Equipment Management, currently when importing medical equipment, importer need to pay attention to 2 points:
- Classification of medical equipment (types A, B, C, D)
- Registration for circulation:
*Note: After having the results of classifying medical equipment as Group 1 (classification A, B) or group 2 (classification C, D): Import procedures will be based on the Medical Equipment classification results.
+ If classified as A or B, make a Declaration of applicable standards;
+ Type C and D (belonging to the list of Medical equipment requiring a license), register for circulation of type C and D Medical equipment;
+ Type to C, D (but not on the list that requires a license), only need to be classified as medical equipment.
Validity of Medical equipment circulation number
The circulation number of medical equipment is valid indefinitely, except in cases where the circulation number of medical equipment is issued according to regulations on emergency issuance of circulation numbers for medical equipment for prevention and control purposes. epidemics, overcoming the consequences of natural disasters and catastrophes.
What certificates are required in importing Medical equipment to Vietnam?
Cases requiring an import license:
a) Medical equipment that does not have a circulation number is imported only to serve scientific research, inspection, testing, testing, quality assessment or training, instructions on use, and instructions on equipment repair medical;
b) Imported medical equipment that does not have a circulation number to meet the urgent needs of disease prevention and control, overcoming the consequences of natural disasters and catastrophes;
c) Medical equipment without a circulation number imported to serve aid or humanitarian aid purposes; gifts and gifts for medical facilities; serving fairs, exhibitions, displays or product introductions;
d) Medical equipment without a circulation number imported to serve humanitarian medical examination and treatment activities;
d) Medical equipment without a circulation number imported for personal medical treatment purposes, including personal medical equipment or according to the special diagnostic needs of a medical facility;
e) Used medical equipment:
– Imported for research and training purposes (do not practice on humans and do not use these medical devices for diagnosis and treatment purposes);
– Temporary import and re-export for display, introduction, participation in fairs and trade exhibitions.
Documents, order and procedures for import, temporary import and re-export of medical equipment comply with the provisions of law on foreign trade management.
List of medical equipment requiring import license
- Imaging diagnostic equipment uses X-rays.
- Magnetic resonance system.
- Diagnostic ultrasound machine.
- Diagnostic endoscopy system.
- Cyclotron system.
- Radioactive isotope diagnostic equipment (PET, PET/CT, SPECT, SPECT/CT systems, iodine concentration measurement equipment I130, I131).
- Refractometer, automatic cornea.
- Electrophysiological measuring machine (Electroencephalograph, Electrocardiograph, Electromyography machine).
- Electroretinometry machine.
- Meter osteoporosis.
- Fundus CT scanner; Fundus fluoroscopy machine.
- Ultrasound fetal heart rate monitor.
- Respiratory function measuring/analyzing machine.
- Biochemical analyzer; Electrolyte and blood gas analyzer.
- Hematology analyzer; Blood type analyzer.
- Coagulation meter; Blood sedimentation rate meter.
- Elisa testing system.
- Cell extraction machine.
- Platelet agglutination meter and function analyzer.
- Machine identifies bacteria and viruses.
- Immunoassay analyzer.
- Reagents, calibrators, in vitro control materials.
- X-ray treatment equipment.
- Endoscopic surgical system.
- Radiation therapy equipment (Cobalt machine for cancer treatment, Linear accelerator for cancer treatment, Gamma scalpels of all kinds, Brachytherapy equipment of all kinds).
- Patient monitor.
- Infusion pump; Electric syringe pump.
- Scalpel (high frequency, laser, ultrasound).
- Surgical microscope.
- Prostate surgical equipment system.
- Heart-lung artificial machine.
- Positioning devices in surgery.
- Cryosurgery equipment.
- Newborn incubators; Baby warmer.Anesthesia/anesthesia machine with breathing.
- Ventilation machine.
- Defibrillator and pacemaker.
- High pressure oxygen chamber.
- Extracorporeal lithotripsy/endoscopic lithotripsy system.
- High-intensity ultrasound equipment system for tumor treatment.
- Blood filtration equipment.
- Specialized ophthalmic surgical systems (Eximer Laser, Phemtosecond Laser, Phaco, Vitreous cutter, Corneal flap cutter).
- Eyeglasses, contact lenses (sightedness, farsightedness, astigmatism) and contact lens preservation solution.
- Laser treatment machine used in ophthalmology.
- Long-term (over 30 days) implanted devices and materials into the body.
- Types of equipment and materials to intervene in the body in the fields of cardiology and cranial nerves.
Customs procedure of import Medical equipment into Vietnam
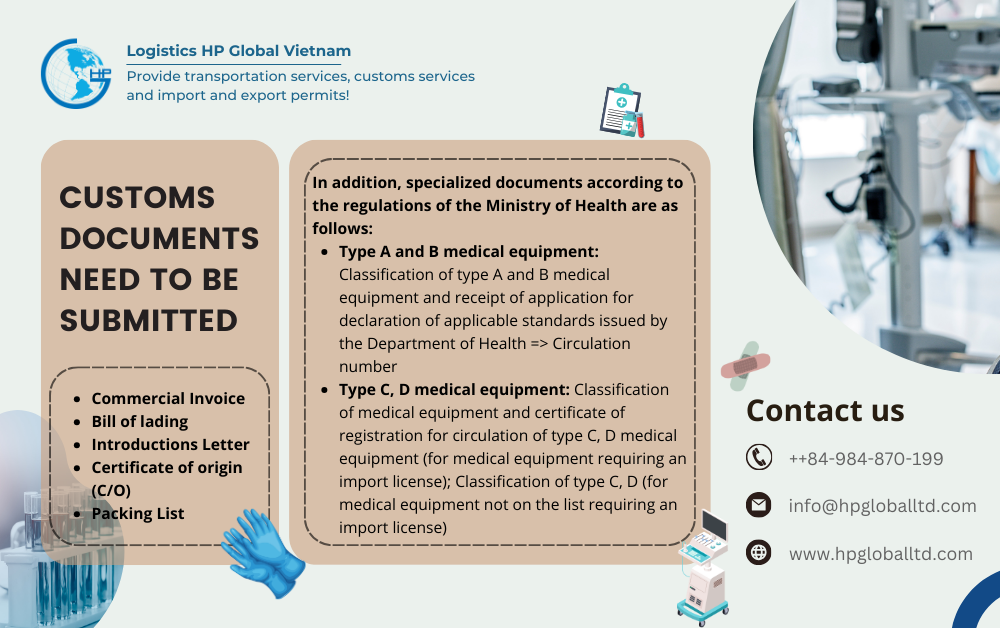
- Commercial Invoice – Copy of the business, with some departments, the original needs to be submitted when the shipment applies special preferential tax with some C/O forms (eg: Form E)
- Bill of lading – Copy of the business
- Letter of introduction – Original copy
- Certificate of origin – Original or electronic copy in case the importer wants to enjoy special preferential import tax
- In some cases, add: Packing List – Copy of the enterprise
- For some branches: add the Customs – Business Partnership Development Agreement – Original copy
- In addition, specialized documents according to regulations of the Ministry of Health are as follows:
Type A and B medical equipment: Classification of type A and B medical equipment and receipt of application for declaration of applicable standards issued by the Department of Health => Circulation number
Type C, D medical equipment: Classification of medical equipment and certificate of registration for circulation of type C, D medical equipment (for medical equipment requiring an import license); Classification C, D (for medical equipment not on the list requiring an import license)
*Note: Due to Decree 98 just took effect, there are still many problems in the process of classification, announcement and registration for circulation. To better understand the actual situation of import and export of medical equipment, please contact us to discuss more details about your shipment → Contact us for inbound and outbound shipment to / from Vietnam – Email: info@hpgloballtd.com
Label for Medical equipment mported to Vietnam:
For medical devices, the current medical device label content is specified in Decree 111/2021/ND-CP
Medical device labels need to show:
a) Circulation number or license number for import of medical equipment;
b) Batch number or serial number of medical equipment;
c) Date of manufacture, expiry date: Sterilized, single-use medical equipment, reagents, calibrators, control materials, and chemicals must have an expiry date. In other cases, write the date of manufacture or expiration date; For medical equipment, machinery and equipment, record the year of manufacture or month and year of manufacture;
d) Warning information, instructions for use, storage instructions, warranty basis: Can be shown directly on the medical equipment label or clearly state instructions for looking up this information on the label. medical equipment.
Note: For goods that are medical equipment produced domestically or imported for circulation in Vietnam, write the name and address of the medical equipment owner and the name and address of the serial number owner. circulate. In case the medical equipment does not have a circulation number, write the name and address of the medical equipment owner and the name and address of the organization or individual on the import license.”;

Select HP Global as the freight forwarder/customs broker for your shipments import Medical equipment into Vietnam

→ Contact us for freight and inbound and outbound services for shipments to/from Vietnam – Email: info@hpgloballtd.com
Logistics HP Global Vietnam
Freight forwarder, Customs Broker and Vietnam Import/export license
Building No. 13, Lane 03, N003, Van Khe, Ha Dong, Hanoi
Website: hpgloballtd.com / hptoancau.com
Email: info@hpgloballtd.com
Phone: ++84 24 73008608 / Hotline: ++84 984870199/ ++84 8 8611 5726/ Whatsapp: ++84 865996476
Note:
– The article is for reference only, prior to using the content, it is suggested that you should contact HP Global for whether any update
– HP Global keep it full right copy right of the article. No copy for commercial purpose is approved.
– Any copy without approval by HP Global (even note quote from website hpgloballtd.com/hptoancau.com) can cause to our claim to google and related agencies.
 Tiếng Việt
Tiếng Việt  English
English  简体中文
简体中文 









