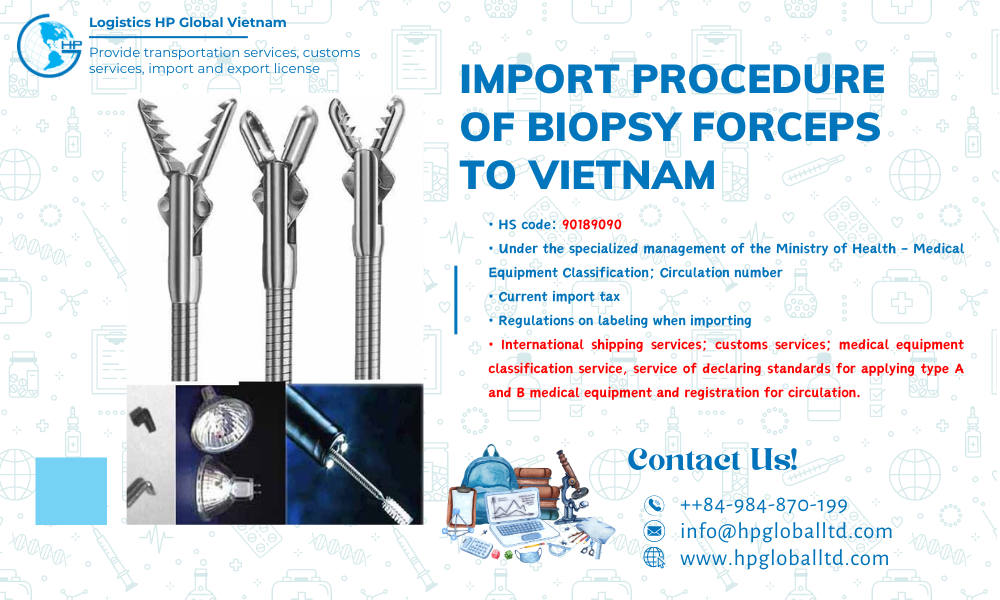
Import Biopsy forceps to Vietnam: Customs procedures; import duty and transportation 2026
Do you want to export Biopsy forceps to Vietnam? Do you need to calculate freight from the export warehouse to Vietnam? You want to know about Vietnam’s procedure and import duties for importing Biopsy forceps to Vietnam? Import procedures for Biopsy forceps?
In this article, Logistics HP Global Vietnam, whose many years in providing freight and logistics services import Biopsy forceps from many countries to Vietnam, will share knowledge and advice on the above issues.
HS code and import duty of Biopsy forceps in Vietnam year 2026
HS Code, Duties, Taxes on importing Biopsy forceps to Vietnam:
Biopsy forceps’s HS code is under Chapter 90: Optical, photographic, cinematographic, measuring, checking, precision, medical or surgical instruments and apparatus; parts and accessories thereof
When importing Biopsy forceps to Vietnam, the importer needs to pay
- Import value-added tax (VAT)
- Import duty
| HS Code | Descriptions | VAT (%) | Preferential Import duty (%) | Normal import tax (%) |
| 9018 | Instruments and appliances used in medical, surgical, dental or veterinary sciences, including scintigraphic apparatus, other electro-medical apparatus and sight-testing instruments | |||
| 90189090 | – – Other | 5 | 0 | 5 |
Note: The HS code and taxes mentioned above are for reference only.
Import duty on Biopsy forceps into Vietnam from Major Markets in 2026
- Biopsy forceps import duty from China to Vietnam: 0% (ACFTA or RCEP)
- Biopsy forceps import duty from India to Vietnam: 0% (AIFTA)
- Biopsy forceps import duty from USA to Vietnam: 0% (Preferential Import duty)
- Biopsy forceps import duty from ASEAN to Vietnam: 0% (ATIGA or RCEP)
- Biopsy forceps import duty from Korea to Vietnam: 0% (AKFTA or VKFTA or RCEP)
- Biopsy forceps import duty from Japan to Vietnam: 0% (AJCEP or VJEPA or RCEP or CPTPP)
- Biopsy forceps import duty from UK to Vietnam: 0% (UKVFTA)
- Biopsy forceps import duty from EU countries to Vietnam:0% (EVFTA)
- Biopsy forceps import duty from Australia to Vietnam: 0% (AANZFTA or RCEP)
- Biopsy forceps import duty from Russia to Vietnam: 0% (EAEUFTA)
- Biopsy forceps import duty from Canada to Vietnam: 0% (CPTPP)
- Biopsy forceps import duty from Mexico to Vietnam: 0% (CPTPP)
The above list is import taxes on Biopsy forceps from some major markets. Note: for countries with FTAs, goods can only enjoy Special preferential import taxes above if they meet the conditions as required by the agreement. If the conditions of the agreement are not met, they will enjoy Preferential import tax.
| If you encounter any difficulties in determining the import tax, please contact us via hotline at ++ 84 886115726 / ++ 84 984870199 or whatsapp: ++84 865996476 for free consultation. |
Governmental management on importing Biopsy forceps to Vietnam
What certificates are required in importing Biopsy forceps to Vietnam?
Biopsy forceps falls under the management of the Ministry of Health. When importing it into Vietnam, it must undergo the classification of Medical Devices. Depending on the result of the medical equipment classification, the following procedures are applied:
- For type A and B medical equipment: Announcement of standards applicable to type A and B medical equipment => Grant of circulation number.
- For medical equipment types C and D: Classification of types C and D + registration for circulation (for items on the list requiring an import license); Just need to do classification C, D (for items not on the list requiring an import license)
In working process, Biopsy forceps are typically classified as Type B.
Customs procedure to import Biopsy forceps into Vietnam:
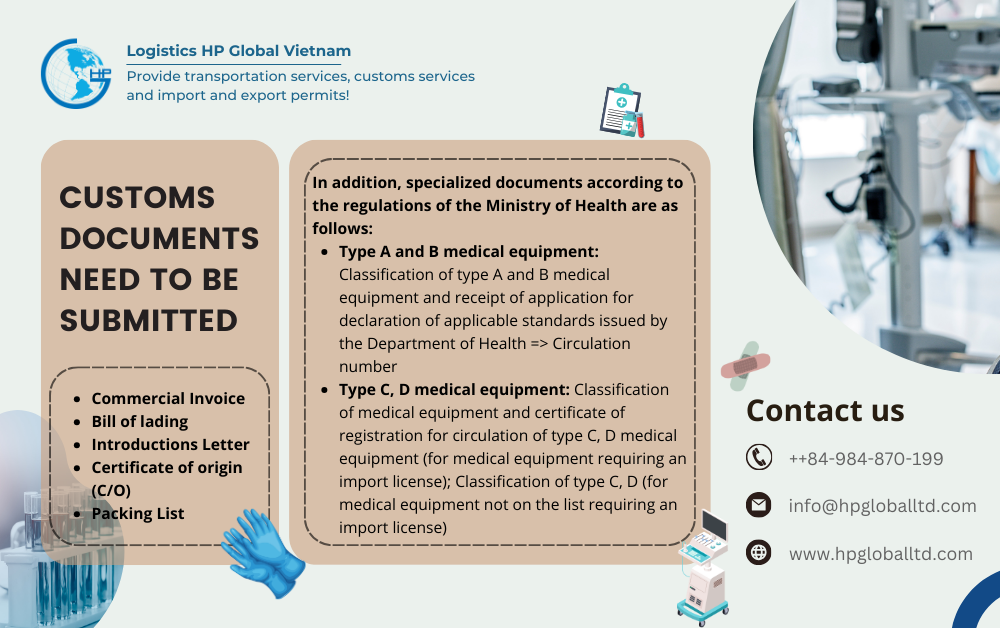
Because of the above management policy, when importing Biopsy forceps into Vietnam, in addition to the standard customs paperwork (Invoice, Bill, Packinglist, Certificate of origin if needed) , Specialized licenses depend on the medical device classification required as follows:
- Type A and B medical equipment: Classification of type A and B medical equipment and receipt of application for declaration of applicable standards issued by the Department of Health => Circulation number
- Type C, D medical equipment: Classification of medical equipment and certificate of registration for circulation of type C, D medical equipment (for medical equipment requiring an import license); Classification of type C, D (for medical equipment not on the list requiring an import license)
Label for Biopsy forceps imported to Vietnam:
Biopsy forceps to imported into Vietnam must comply with regulations regarding labeling for imported goods (Name of goods; Name and address of the organization/individual responsible for the goods; Origin of the goods, Other contents according to the nature of each type of goods)
Labeling requirements for medical devices include:
- Circulation number or import license number of the medical device.
- Lot number or serial number of the medical device.
- Date of manufacture and expiration date: For sterilized medical devices, single-use devices, test kits, standards, control materials, and chemicals, the expiration date must be specified. For other cases, the date of manufacture or expiration date should be provided. For medical devices that are machinery or equipment, the year of manufacture or month and year of manufacture should be stated.
- Warning information, instructions for use, storage instructions, warranty information: This information can be directly displayed on the label of the medical device or clearly indicate how to access this information on the label of the medical device.

Freight Shipping For Biopsy forceps To Vietnam
Shipping time by sea and by air
To check the specific international shipping times by port or airport, you can send a message or call the phone/Zalo number ++84 886115726 – ++84 984870199, whatsapp: ++84 865996476; email: info@hpgloballtd.com
Shipping freight
Freight charges depend on many factors, both fixed and variable over time. Therefore, please provide specific or anticipated shipment details to HP Global Vietnam to receive a complete quote on all costs for the entire import process. – Contact: ++84 886115726 or ++84984870199, whatsapp: ++84 865996476, email: info@hpgloballtd.com
Procedures for Importing Certain Similar Products
| Commodity | Related link |
| Diagnostic chemicals for PCR | Import procedures for Diagnostic chemicals for PCR to Vietnam |
| Chemicals used for coagulation analyzers | Import Duty And Procedures For Chemicals used for coagulation analyzers To Vietnam |
| Patient monitor | Import duty and procedures for Patient monitor to Vietnam |
| Coronary stent | Import procedures for Coronary stent to Vietnam |
| Linear accelerator | Import Duty And Procedures For Linear accelerator To Vietnam |
Select HP Global as the freight forwarder/customs broker for your shipments import Biopsy forceps into Vietnam
HP Global is a freight forwarder with a top reputation in Vietnam.

→ Contact us for freight and inbound and outbound services for shipments to/from Vietnam – Email: info@hpgloballtd.com
Logistics HP Global Vietnam
Freight forwarder, Customs Broker and Vietnam Import/export license
Building No. 13, Lane 03, N003, Van Khe, Ha Dong, Hanoi
Website: hpgloballtd.com / hptoancau.com
Email: info@hpgloballtd.com
Phone: ++84 24 73008608 / Hotline: ++84 984870199/ ++84 8 8611 5726/ Whatsapp: ++84 865996476
Note:
– The article is for reference only, prior to using the content, it is suggested that you should contact HP Global for whether any update
– HP Global keep it full right copy right of the article. No copy for commercial purpose is approved.
– Any copy without approval by HP Global (even note quote from website hpgloballtd.com/hptoancau.com) can cause to our claim to google and related agencies.
 Tiếng Việt
Tiếng Việt  English
English  简体中文
简体中文