Import Chewable tablets to Vietnam: Customs procedures; import duty and transportation 2026
You want to export Chewable tablets to Vietnam? You need to calculate freight from export warehouse to Vietnam? You want to know about Vietnam’s procedure and import duties on importing Chewable tablets to Vietnam? Import procedures for Chewable tablets?
In this article, Logistics HP Global Vietnam, whose many years in providing freight and logistics services import Chewable tablets from many countries to Vietnam, will share knowledge and advise on above issues.

HS code and import duty of Chewable tablets in Vietnam
HS code of Chewable tablets in Vietnam
In importing/exporting any commodity, In order to properly determine duties, policies, procedures of the commodity, one of the first thing needed to determine is the HS code of the commodity
Chewable tablets under Chapter 30 – PHARMACEUTICAL PRODUCTS
VAT and preferential import duty on Chewable tablets to Vietnam in 2026
| HS Code | Describe | VAT (%) | Preferential import tax (%) | Normal import tax (%) |
| 3004 | Medicaments (excluding goods of heading 30.02, 30.05 or 30.06) consisting of mixed or unmixed products for therapeutic or prophylactic uses, put up in measured doses (including those in the form of transdermal administration systems) or in forms or packings for retail sale. | |||
| 30049059 | – – – Other | 5 | 3 | 4.5 |
Note: The HS code and taxes mentioned above are for reference only.
Import duty on Chewable tablets into Vietnam from Major Markets in 2026
- Chewable tablets import duty from China to Vietnam: 0% (ACFTA or RCEP)
- Chewable tablets import duty from India to Vietnam: 5% (AIFTA)
- Chewable tablets import duty from USA to Vietnam: 3% (Preferential Import duty)
- Chewable tablets import duty from ASEAN to Vietnam: 0% (ATIGA or RCEP)
- Chewable tablets import duty from Korea to Vietnam: 0% (AKFTA or VKFTA or RCEP)
- Chewable tablets import duty from Japan to Vietnam: 0% (AJFTA or VJFTA or RCEP or CPTPP)
- Chewable tablets import duty from UK to Vietnam: 0% (UKVFTA)
- Chewable tablets import duty from EU countries to Vietnam: 0% (EVFTA)
- Chewable tablets import duty from Australia to Vietnam: 0% (AANZFTA or RCEP)
- Chewable tablets import duty from Russia to Vietnam: 0% (VN-EAEUFTA)
- Chewable tablets import duty from Canada to Vietnam: 0% (CPTPP)
- Chewable tablets import duty from Mexico to Vietnam: 0% (CPTPP)
The above list is import taxes on Chewable tablets from some major markets. Note: for countries with FTAs, goods can only enjoy Special preferential import taxes above if they meet the conditions as required by the agreement. If the conditions of the agreement are not met, they will enjoy Preferential import tax.
| If you encounter any difficulties in determining the import tax, please contact us via hotline at ++ 84 886115726 / ++ 84 984870199 or whatsapp: ++84 865996476 for free consultation. |
Governmental management on importing Chewable tablets to Vietnam
What certificates are required in importing Chewable tablets to Vietnam?
Chewable tablets falls under the management of the Ministry of Health. When importing it into Vietnam, it must undergo the classification of Medical Devices. Depending on the result of the medical equipment classification, the following procedures are applied:
- For type A and B medical equipment: Announcement of standards applicable to type A and B medical equipment => Grant of circulation number.
- For medical equipment types C and D: Classification of types C and D + registration for circulation (for items on the list requiring an import license); Just need to do classification C, D (for items not on the list requiring an import license)
In working process, Chewable tablets are typically classified as Type D.
Customs procedure to import Chewable tablets into Vietnam:
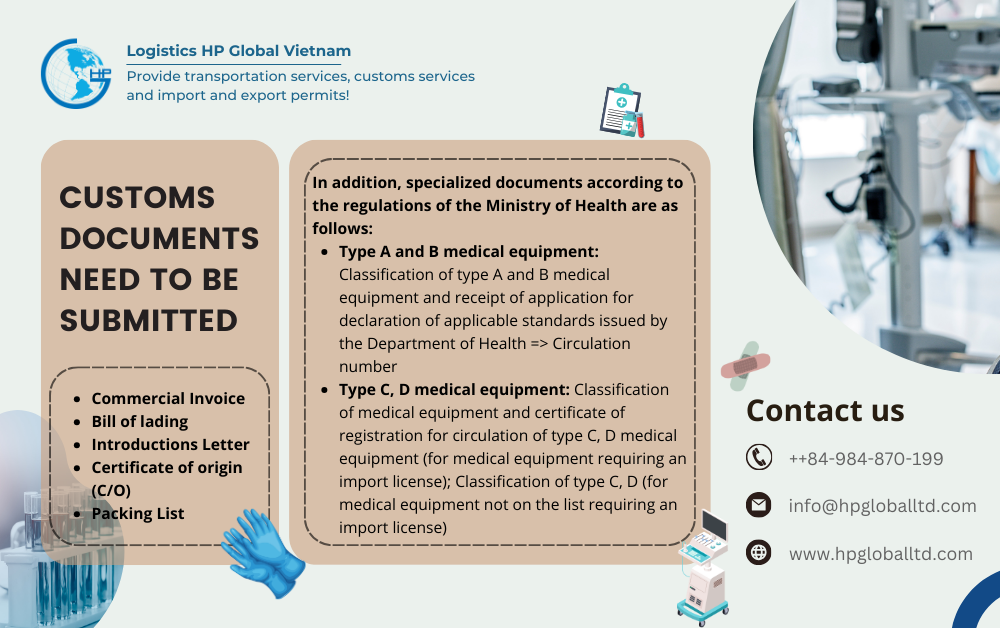
Because of the above management policy, when importing Chewable tablets into Vietnam, in addition to the standard customs paperwork (Invoice, Bill, Packinglist, Certificate of origin if needed) , Specialized licenses depend on the medical device classification required as follows:
- Type A and B medical equipment: Classification of type A and B medical equipment and receipt of application for declaration of applicable standards issued by the Department of Health => Circulation number
- Type C, D medical equipment: Classification of medical equipment and certificate of registration for circulation of type C, D medical equipment (for medical equipment requiring an import license); Classification of type C, D (for medical equipment not on the list requiring an import license)
Label for Chewable tablets imported to Vietnam:
Chewable tablets to imported into Vietnam must comply with regulations regarding labeling for imported goods (Name of goods; Name and address of the organization/individual responsible for the goods; Origin of the goods, Other contents according to the nature of each type of goods)
Labeling requirements for medical devices include:
- Circulation number or import license number of the medical device.
- Lot number or serial number of the medical device.
- Date of manufacture and expiration date: For sterilized medical devices, single-use devices, test kits, standards, control materials, and chemicals, the expiration date must be specified. For other cases, the date of manufacture or expiration date should be provided. For medical devices that are machinery or equipment, the year of manufacture or month and year of manufacture should be stated.
- Warning information, instructions for use, storage instructions, warranty information: This information can be directly displayed on the label of the medical device or clearly indicate how to access this information on the label of the medical device.

Freight Shipping For Chewable tablets To Vietnam
Shipping time by sea and by air
To check the specific international shipping times by port or airport, you can send a message or call the phone/Zalo number ++84 886115726 – ++84 984870199, whatsapp: ++84 865996476; email: info@hpgloballtd.com
Shipping freight
Freight charges depend on many factors, both fixed and variable over time. Therefore, please provide specific or anticipated shipment details to HP Global Vietnam to receive a complete quote on all costs for the entire import process. – Contact: ++84 886115726 or ++84984870199, whatsapp: ++84 865996476, email: info@hpgloballtd.com
Procedures for Importing Certain Similar Products
| Commodity | Related link |
| Throat spray solution | Import Procedure of Throat spray solution to Vietnam |
| Disintegration Tester | Import Duty And Procedures For Disintegration Tester To Vietnam |
| Oxygen mask | Procedures and Import Duty for Oxygen mask into Vietnam |
| Dentures | Import Duty And Procedures For Dentures To Vietnam |
Select HP Global as the freight forwarder/customs broker for your shipments import Chewable tablets into Vietnam
HP Global is a freight forwarder with a top reputation in Vietnam.

→ Contact us for freight and inbound and outbound services for shipments to/from Vietnam – Email: info@hpgloballtd.com
Logistics HP Global Vietnam
Freight forwarder, Customs Broker and Vietnam Import/export license
Building No. 13, Lane 03, N003, Van Khe, Ha Dong, Hanoi
Website: hpgloballtd.com / hptoancau.com
Email: info@hpgloballtd.com
Phone: ++84 24 73008608 / Hotline: ++84 984870199/ ++84 8 8611 5726/ Whatsapp: ++84 865996476
Note:
– The article is for reference only, prior to using the content, it is suggested that you should contact HP Global for whether any update
– HP Global keep it full right copy right of the article. No copy for commercial purpose is approved.
– Any copy without approval by HP Global (even note quote from website hpgloballtd.com/hptoancau.com) can cause to our claim to google and related agencies.
 Tiếng Việt
Tiếng Việt  English
English  简体中文
简体中文